Select the true statements about the electron transport chain – The electron transport chain (ETC) plays a pivotal role in cellular respiration, a fundamental process that generates energy for cells. Understanding the ETC’s structure, components, and mechanisms is crucial for comprehending energy production at the cellular level.
This exploration delves into the intricate workings of the ETC, unraveling its role in creating a proton gradient across the mitochondrial membrane and harnessing this gradient to drive ATP synthesis. Along the way, we uncover the interplay between electron carriers, the significance of cytochrome c, and the factors influencing the efficiency of chemiosmosis.
Electron Transport Chain (ETC)
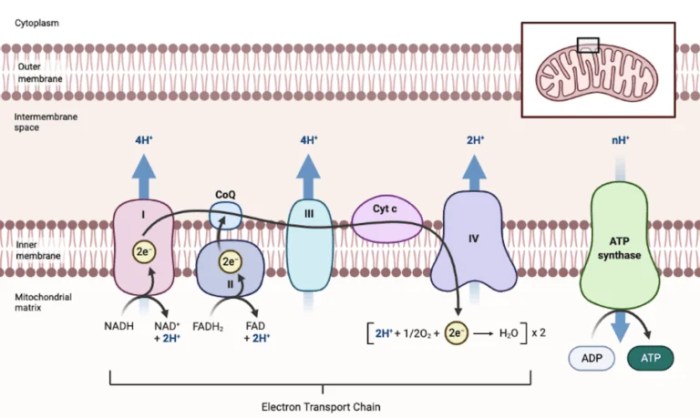
The electron transport chain (ETC) is a series of protein complexes located in the inner mitochondrial membrane. It plays a crucial role in cellular respiration, the process by which cells generate energy in the form of ATP.
The ETC consists of four protein complexes (I-IV) and two mobile electron carriers (ubiquinone and cytochrome c). Electrons from NADH and FADH2 are passed along the ETC, releasing energy that is used to pump protons across the mitochondrial membrane. This creates a proton gradient, which is used by ATP synthase to generate ATP.
Structure and Components of the ETC
The ETC is composed of four protein complexes (I-IV) and two mobile electron carriers (ubiquinone and cytochrome c).
- Complex I (NADH dehydrogenase): Receives electrons from NADH and passes them to ubiquinone.
- Complex II (succinate dehydrogenase): Receives electrons from FADH2 and passes them to ubiquinone.
- Complex III (cytochrome bc1 complex): Transfers electrons from ubiquinone to cytochrome c.
- Complex IV (cytochrome c oxidase): Transfers electrons from cytochrome c to oxygen, the final electron acceptor.
Electron Carriers
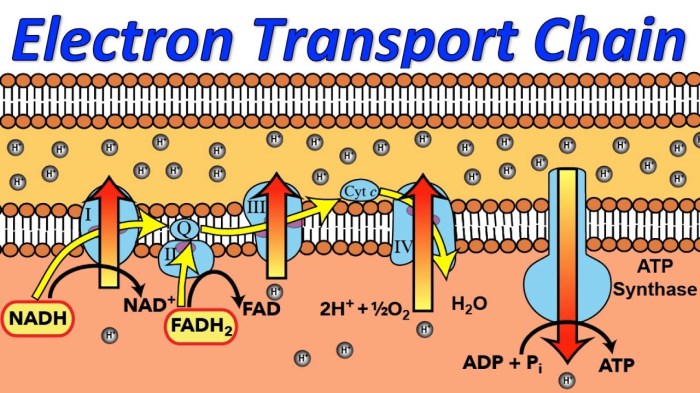
Electrons are transferred along the ETC by two mobile electron carriers: ubiquinone and cytochrome c.
Comparison of NADH and FADH2
| Characteristic | NADH | FADH2 |
|---|---|---|
| Electron donor | NAD+ | FAD |
| Number of electrons transferred | 2 | 2 |
| Energy released per electron pair | More | Less |
Role of Cytochrome c in Electron Transfer, Select the true statements about the electron transport chain
Cytochrome c is a small, water-soluble protein that plays a crucial role in electron transfer between Complex III and Complex IV. It is located in the intermembrane space of the mitochondria and transfers electrons from Complex III to Complex IV via a redox reaction.
Proton Gradient: Select The True Statements About The Electron Transport Chain
The ETC creates a proton gradient across the mitochondrial membrane by pumping protons from the mitochondrial matrix to the intermembrane space.
Role of ATP Synthase in Utilizing the Proton Gradient
ATP synthase is a protein complex located in the inner mitochondrial membrane. It utilizes the proton gradient to generate ATP from ADP and inorganic phosphate.
- As protons flow down the proton gradient through ATP synthase, they cause the rotation of a subunit within the enzyme.
- This rotation drives conformational changes in the enzyme that result in the synthesis of ATP.
Chemiosmosis
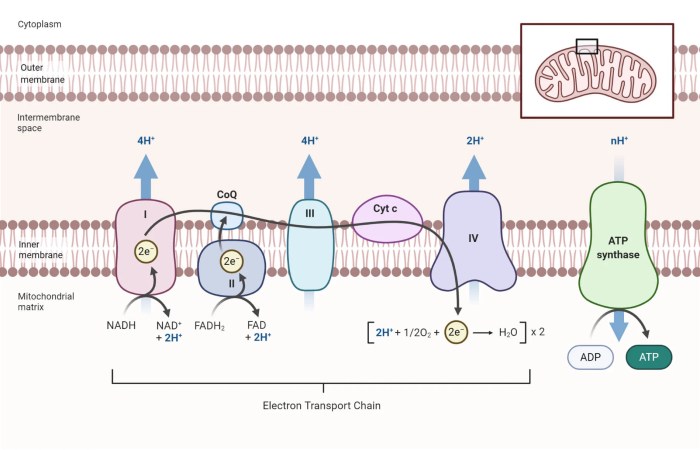
Chemiosmosis is the process by which the ETC uses the proton gradient to generate ATP. The proton gradient provides the energy to drive the synthesis of ATP by ATP synthase.
Factors that Can Affect the Efficiency of Chemiosmosis
- Proton leak: Protons can leak back across the mitochondrial membrane, reducing the efficiency of chemiosmosis.
- Uncoupling proteins: These proteins can dissipate the proton gradient, reducing the efficiency of chemiosmosis.
- pH gradient: The pH gradient across the mitochondrial membrane can affect the efficiency of chemiosmosis.
Regulation of the ETC
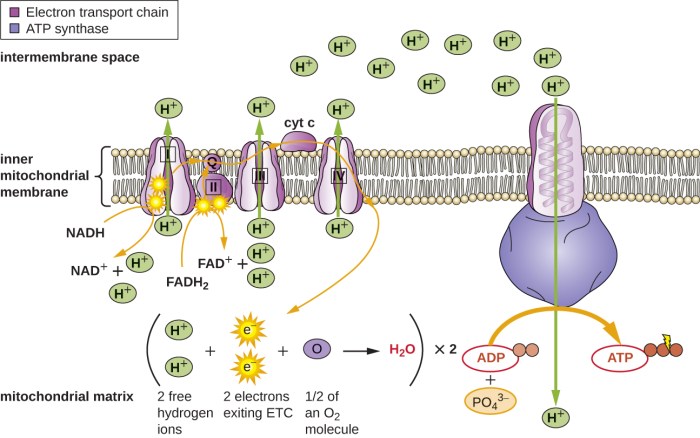
The activity of the ETC is regulated by a number of factors, including:
- ATP levels: High levels of ATP inhibit the activity of the ETC.
- NADH/NAD+ ratio: A high NADH/NAD+ ratio stimulates the activity of the ETC.
- Oxygen levels: The ETC requires oxygen as the final electron acceptor. Low oxygen levels can inhibit the activity of the ETC.
Integration of the ETC with Other Metabolic Pathways
The ETC is integrated with other metabolic pathways, including glycolysis, the citric acid cycle, and fatty acid oxidation. These pathways provide the ETC with the electrons that are used to generate ATP.
Answers to Common Questions
What is the primary function of the electron transport chain?
The electron transport chain’s primary function is to generate a proton gradient across the mitochondrial membrane, which is then utilized by ATP synthase to produce ATP.
How does the electron transport chain contribute to ATP production?
The electron transport chain creates a proton gradient across the mitochondrial membrane, and this gradient drives the movement of protons through ATP synthase, leading to the synthesis of ATP.
What is the role of cytochrome c in the electron transport chain?
Cytochrome c is a mobile electron carrier that transfers electrons between complexes III and IV of the electron transport chain.